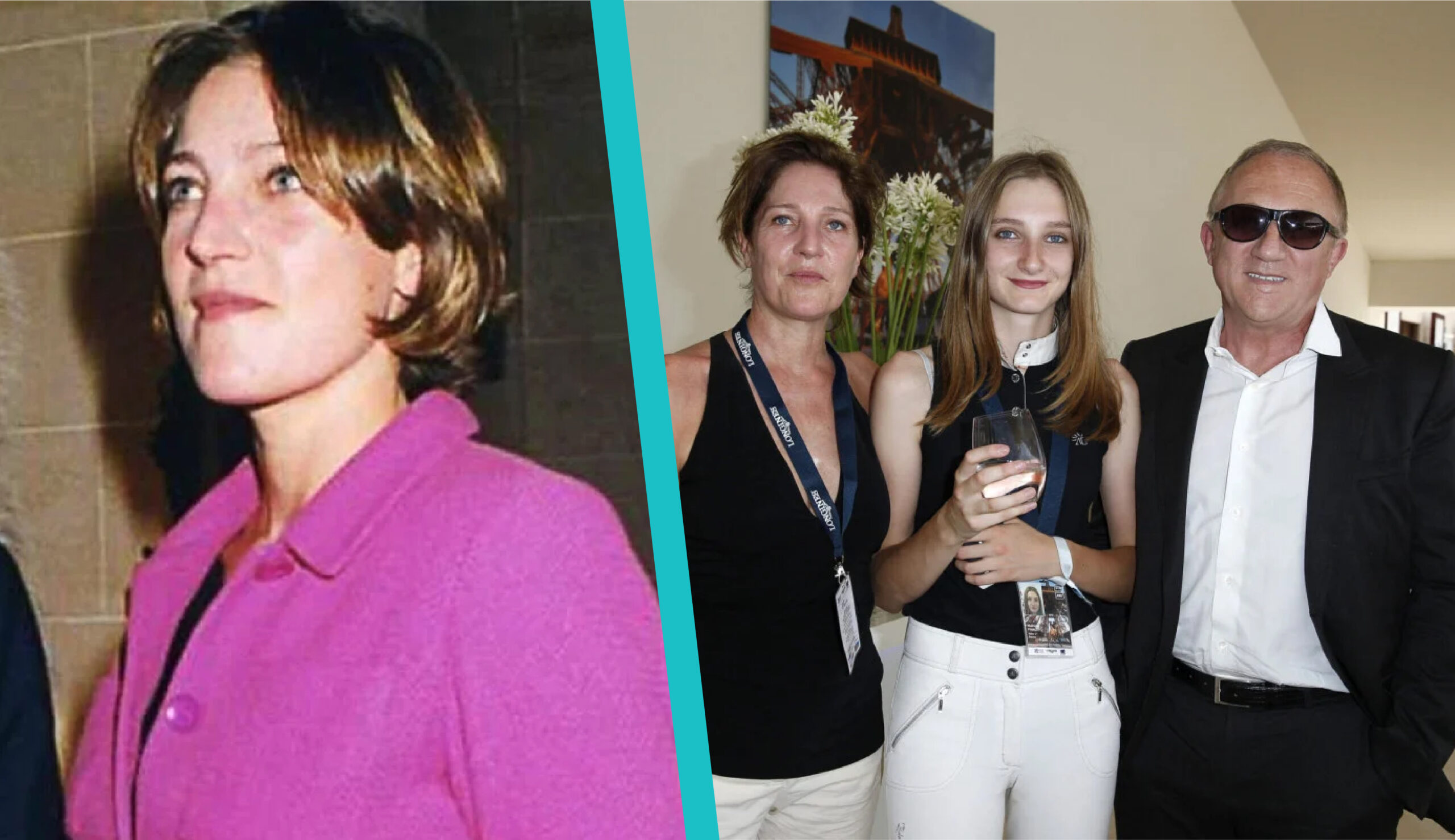
Dorothée Lepère is best known as the first wife of French billionaire François-Henri Pinault, the CEO of Kering. Though she lives a private life, her name is often mentioned in relation to Pinault’s powerful position in fashion and luxury, as well as his marriage to actress Salma Hayek. Dorothée is also the mother of two children, François and Mathilde, who are both connected to the fashion and luxury world.
Quick Bio Table
| Detail | Information |
| Full Name | Dorothée Lepère |
| Known For | First wife of François-Henri Pinault |
| Nationality | French |
| Profession | Interior Designer |
| Marriage Year | 1996 |
| Divorce Year | 2004 |
| Ex-Husband | François-Henri Pinault |
| Children | François Pinault Jr., Mathilde Pinault |
| Daughter’s Birth Year | 2001 |
| Son’s Birth Year | 1998 |
| Current Status | Private Life |
| Connection | Pinault Family Legacy |
| Relation to Salma Hayek | Mother of Salma’s stepchildren |
Early Life of Dorothée Lepère
The early background of Dorothée Lepère is mostly private. Unlike other celebrity partners, she has chosen to stay away from the spotlight. Reports suggest she was born in France and developed her career as an interior designer, a path that highlights her creative and professional side. Even though her personal childhood details remain unknown, her later life became tied to one of the most influential families in Europe.
Marriage to François-Henri Pinault
In 1996, Dorothée Lepère married François-Henri Pinault. At that time, Pinault was already an important figure in his family’s company, which would later become the global luxury group Kering. Their marriage lasted until 2004. The couple’s relationship brought attention to Dorothée, as she was suddenly connected to one of France’s most powerful business families.
Children of Dorothée Lepère and François-Henri Pinault
During their marriage, Dorothée Lepère and Pinault welcomed two children. Their son François was born in 1998, and their daughter Mathilde was born in 2001. Mathilde Pinault has since become known for her role in the fashion world and as an equestrian athlete. She often attends events with her father and stepmother, Salma Hayek, while Dorothée continues to support her children away from the public eye.
People Read Also: Discover Genevieve Mecher: All About Jen Psaki’s Firstborn
Life After Divorce
After the divorce in 2004, Dorothée Lepère chose to live a low-key and independent life. While her ex-husband went on to marry Salma Hayek in 2009, Dorothée avoided public scandals and focused on her family and career. This choice reflects her preference for privacy, even though her children remain in the public’s view due to their father’s fame.
Career as an Interior Designer
Dorothée Lepère is often described as a professional interior designer. This career shows her artistic side and her ability to work in a creative field separate from her husband’s business empire. Although details about her work are not heavily published, it is clear she values building her own identity outside of her marriage.
Connection With the Pinault Legacy
The name Pinault is tied to one of the strongest fashion dynasties in the world. Through her marriage, Dorothée Lepère became part of this legacy. Even though she later separated from Pinault, she remains linked to this history through her children. Her daughter Mathilde is especially visible, attending fashion shows by brands owned by the Kering group.
Relationship With Salma Hayek and Family
After the divorce, François-Henri Pinault married Salma Hayek. Together they share a daughter, Valentina Paloma Pinault. Despite these changes, Dorothée’s children grew close to their father’s new family. Public events show that Mathilde often appears alongside Salma Hayek, proving that the family dynamic is stable and respectful. Dorothée’s role remains more private, but her influence as their mother is still very important.
Private and Personal Life
Unlike many celebrity spouses, Dorothée Lepère has successfully kept her private life away from media attention. She does not appear often in interviews or public magazines. This decision has allowed her to protect her identity while still supporting her children’s growth in the fashion and sports industries.
Influence on Her Children
It is clear that Dorothée Lepère had a big impact on her children’s upbringing. François Pinault Jr. and Mathilde Pinault continue to represent both their parents in public events. While François lives mostly away from media, Mathilde has become an admired figure in equestrian sports and luxury fashion. Dorothée’s parenting is often credited with giving them balance between fame and normal life.
Legacy of Dorothée Lepère
Though she is not a celebrity, Dorothée Lepère holds an important place in the story of François-Henri Pinault. As his first wife and the mother of his first children, her influence continues through the next generation. She represents the private side of a family that is often under public attention. Her life shows dignity, independence, and strength in staying true to her own path.
More Stories: HarmoniCode com: Your Gateway to Coding Success
Conclusion
The story of Dorothée Lepère is more than being the ex-wife of François-Henri Pinault. It is about a woman who balanced her role as a mother, interior designer, and private individual in the middle of one of the world’s most powerful families. She remains an important figure through her children and her quiet strength. Even with the fame around her, she has shown that a private and stable life is still possible.
FAQs
1. Who is Dorothée Lepère?
She is the first wife of François-Henri Pinault and the mother of his two children.
2. What is Dorothée Lepère’s profession?
She is described as an interior designer.
3. How many children does she have?
She has two children, François and Mathilde.
4. When was Dorothée Lepère married to Pinault?
They were married from 1996 to 2004.
5. Is Dorothée Lepère in the public spotlight today?
No, she lives a private life and avoids media attention.